A surge in glucose activity precedes gastrulation in mice embryos, according to a new study from Yale University researchers published in Nature (2024). This research reveals that glucose metabolism is essential for directing early embryonic development by regulating vital cell signaling during critical phases of embryogenesis.
The paper, titled "Selective utilization of glucose metabolism guides mammalian gastrulation," identifies two distinct phases of glucose utilization during mouse gastrulation.
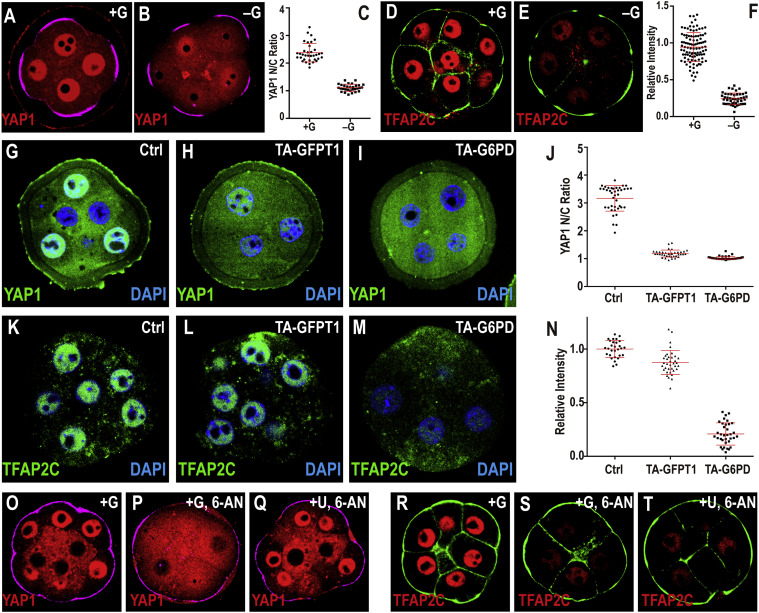
In the first phase, glucose is processed through the hexosamine biosynthetic pathway (HBP) in epiblast cells, which aids in the synthesis of proteoglycans that are vital for fibroblast growth factor (FGF) signaling. This signaling is important for cell differentiation and the elongation of the primitive streak, a structure that eventually gives rise to the neural plate and the spinal cord.
The second phase shifts glucose through glycolysis, supplying energy and metabolites crucial for the migration and lateral expansion of mesodermal cells.
The researchers mapped glucose uptake's spatial and temporal patterns using single-cell-resolution imaging of developing mouse embryos, stem cell models, and embryo-derived tissues. They found that the initial wave of glucose metabolism occurs in the posterior epiblast cells and progresses forward as development continues. The later glycolytic wave supports mesodermal cell migration away from the primitive streak, enhancing lateral expansion.
To validate the involvement of these metabolic pathways, the team inhibited glucose metabolism with chemical blockers, demonstrating that this disruption affected both primitive streak formation and mesoderm specification. Specifically, targeting the HBP hindered primitive streak development, while inhibiting late-stage glycolysis impaired mesodermal cell migration without affecting initial cell fate decisions.
Additionally, the study shows that glucose metabolism affects extracellular signal-regulated kinase (ERK) signaling pathways, which are crucial for cell differentiation and movement during gastrulation. Inhibition of glucose metabolism led to reduced ERK activity, whereas adding N-acetylglucosamine—derived from the HBP—restored ERK signaling and mitigated developmental defects.
Further analysis with stem cell-based embryo models and mesoderm explants confirmed the HBP's critical role in epiblast cell fate transitions and highlighted glycolysis' support for mesodermal cell migration.
RNA sequencing of mesoderm explants treated to inhibit glycolysis or ERK signaling revealed a downregulation of pathways essential for cell migration and extracellular matrix interactions.
These findings underscore that glucose metabolism, in concert with genetic and signaling mechanisms, plays a vital role in the successful patterning and morphogenesis of the developing embryo. This research redefines the perception of cellular metabolism from a mere background function to a central orchestrator of embryonic development





















0 Comments