The placenta is an extraordinary organ, essential for sustaining life during gestation, yet it remains one of the least understood in human biology. From the earliest stages of pregnancy, the placenta forms a lifeline between the developing fetus and the mother, taking on multiple roles: it functions as a lung by exchanging oxygen and carbon dioxide, as a kidney by filtering waste, as an immune system by protecting the fetus, and as a digestive system by providing nutrients. The placenta is the first organ to develop in an embryo, highlighting its importance, yet scientific understanding of its complex biology and development has lagged.
The placenta’s role extends far beyond basic support functions; it is an arena where evolutionary and genetic forces play out. The organ contains both maternal and paternal DNA, and as a result, it reflects a balance of genetic interests: paternal genes may push for maximizing fetal growth, while maternal genes aim to conserve resources, preventing excessive demands on her body. Researchers refer to this dynamic as an “evolutionary battleground,” a metaphor for the subtle tug-of-war over nutrient allocation and fetal development.
The health implications of abnormal placental development are profound, as seen in conditions collectively known as “great obstetric syndromes.” Among these is preeclampsia, a complication marked by high blood pressure and protein in the urine that can be life-threatening for both mother and child. Despite its prevalence, affecting 3-5% of pregnancies worldwide, the only definitive treatment for preeclampsia remains delivery of the baby and placenta, regardless of gestational age. This lack of treatments highlights the knowledge gap surrounding placental biology—a gap rooted in limited research models and ethical constraints on studying human placental tissue.
Studying the placenta has been especially challenging due to its species-specific evolution, as its structure, invasion of the uterine wall, and function differ significantly across mammals. While animal models have provided foundational insights, they can only partially mimic the human placental experience. These differences have spurred researchers to turn to in vitro, or lab-based, placental models in recent years. Advances in stem cell and cell culture technologies now enable researchers to grow human placental cells in the lab, offering new ways to observe the organ’s development and functions in a controlled environment.
Six days after fertilization, the human embryo holds epiblast cells (red) and the trophectoderm (green). Epiblast cells go on to form the fetus, while the trophectoderm gives rise to the placenta.
These breakthroughs in cell culture provide a promising path forward, as scientists seek to replicate the complexities of the placenta outside the womb. As researchers continue to unlock its mysteries, they may find answers to treat pregnancy complications that have puzzled obstetricians for centuries, further illuminating the “space between” mother and child. This pioneering work underscores the placenta’s unique role in human life—a gateway to understanding both the origins of life and the protective systems that sustain it.
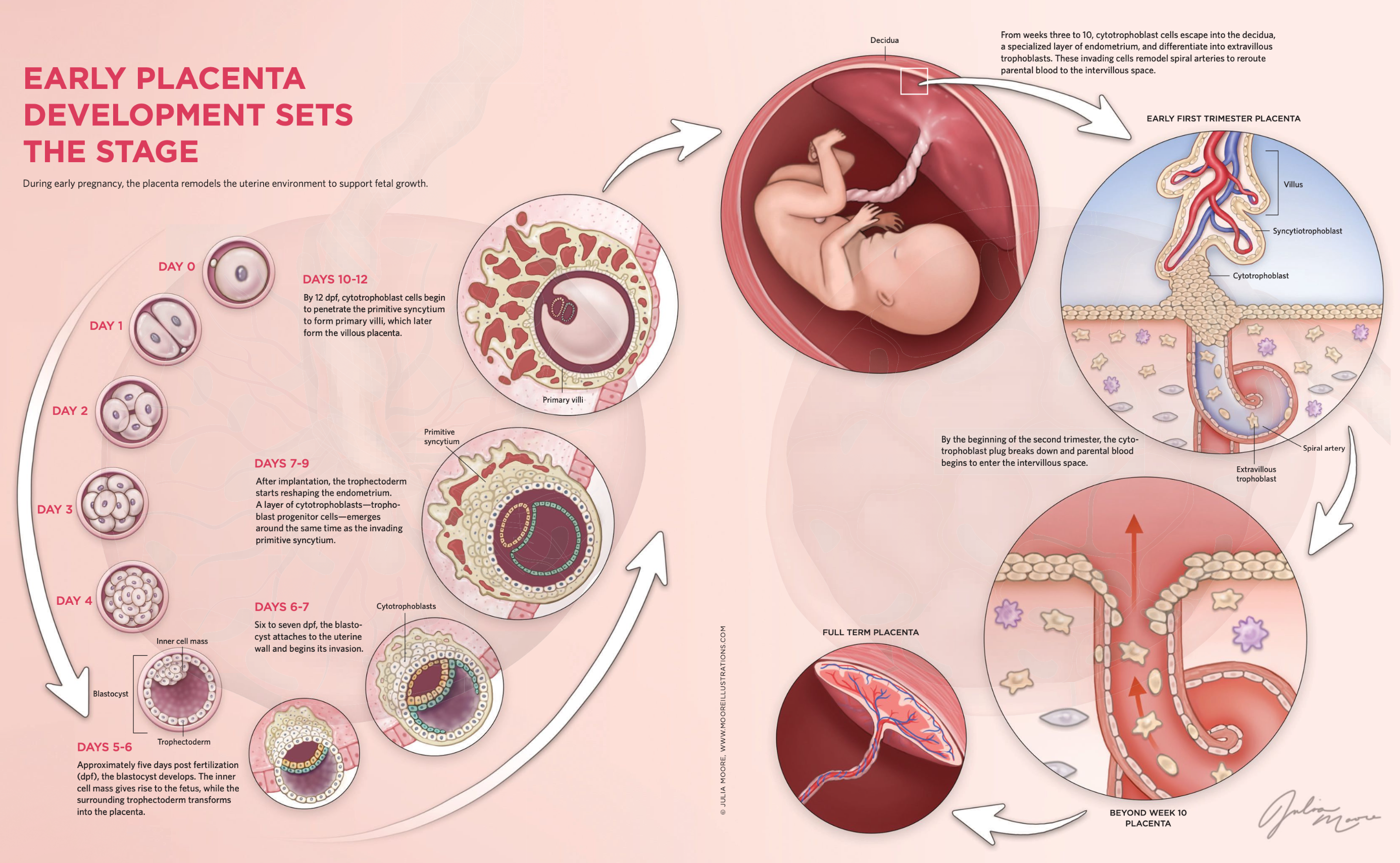
The journey of a single fertilized cell to a complex, organized structure like the blastocyst is not just a feat of biological engineering but also a key window into human health and development. Around six days post-fertilization, the fertilized zygote becomes a blastocyst, containing an inner cell mass (ICM) that will form the fetus and a layer of outer cells known as the trophectoderm, which later develops into the placenta. This early stage is a mysterious and powerful period where the foundations for nearly every organ and system in the body are laid. Scientists like Norah Fogarty are keen to unlock the secrets of this "black box" phase, particularly to understand how cellular pathways and molecular signals drive differentiation and set the trajectory for healthy or, conversely, compromised fetal development.
Fogarty's interest in these early stages of life grew from her studies in molecular medicine. By focusing on how fetal health can influence lifelong well-being, she became interested in how developmental issues in utero may contribute to adult diseases. The field of fetal origins of adult disease, a concept advanced in the 1990s, asserts that conditions in the womb—nutrition, stress, and cellular health—can predispose individuals to diseases such as diabetes, cardiovascular issues, and mental health disorders later in life. This idea has helped reframe pregnancy research, emphasizing how the first weeks after conception can impact health decades down the line.
As she pursued her Ph.D. at the University of Cambridge, Fogarty explored the molecular mechanisms involved in placental development. The placenta, a temporary organ, is crucial for nutrient exchange between the mother and fetus and plays a critical role in regulating fetal development. However, surprisingly little is known about how it forms, largely because of ethical and technical constraints in studying human embryos beyond certain stages. Placental cells are highly specialized, and until recently, researchers struggled to grow accurate human models to study this process.
One of the most promising discoveries for this line of research came in 2018 when scientists succeeded in creating human trophoblast stem cells (hTSCs). These cells, derived from early-stage placental cells, can mimic aspects of placental development in vitro and be induced to differentiate into various trophoblast subtypes. Unlike previous models, hTSCs are genetically and functionally closer to actual human placental cells, offering a powerful tool to study early development and disorders such as preeclampsia—a potentially dangerous condition linked to placental malfunction that can affect the mother's organs and lead to complications in the fetus.
Research into the specific transcription factors that drive these early developmental processes, such as OCT4 and CDX2, is essential to understanding how and when placental cells begin their specialization. Using gene-editing technologies like CRISPR-Cas9, Fogarty and her colleagues are able to analyze the effects of specific genes on cell differentiation. In particular, they have uncovered notable differences in how these genes operate in human and mouse embryos, highlighting why models using human cells are so vital.
As Fogarty and her team probe deeper into these early developmental signals, they hope to identify the origins of placental diseases at the cellular level. Understanding these processes could one day enable doctors to detect potential issues in early pregnancy or even develop interventions to support a healthy placenta. By focusing on these critical early stages of life, Fogarty's work brings us closer to understanding how the first days after conception shape the health of a lifetime.
The development of the placenta is a finely tuned, complex process that has evolved to balance the often competing needs of the parent and the fetus. During early pregnancy, the placenta begins forming from clusters of cells that eventually become villi, tree-like structures that bring fetal and parental blood supplies into close contact. These villi house the fetal capillary system, enabling nutrient and gas exchange essential for fetal development. Key to this process are the cytotrophoblasts, cells that differentiate and migrate deeper into the uterine wall to become extravillous trophoblasts (EVTs), which remodel maternal arteries to increase blood flow to the placenta. This process, which culminates around 10–12 weeks into pregnancy, allows the parental blood supply to fully connect to the growing placenta, setting the stage for fetal growth.
The uterine wall, however, is more than a passive environment. It contains immune cells, particularly unique uterine natural killer (NK) cells, which play a critical role in moderating the interaction between the maternal and fetal cells. Unlike NK cells in the blood, uterine NK cells are adapted to balance immune tolerance with protection. This balance is crucial, as the fetus carries genetic material from the other parent, making it genetically "foreign" to the mother. This delicate interplay between immune tolerance and defense mechanisms has led some researchers to describe pregnancy as a form of "controlled invasion," in which the placenta draws resources without triggering a full immune response.
Dr. Ashley Moffett's work in reproductive immunology has been instrumental in understanding these unique immune cells. Her career in this field began unexpectedly in the 1980s when, without initial interest in obstetrics, she took a position at a maternity hospital in Cambridge. This experience exposed her to unsolved problems like preeclampsia, a pregnancy disorder partly resulting from insufficient EVT invasion into the uterine wall. Intrigued by unusual cells in the uterus that resembled NK cells, Moffett collaborated with her former professor, Dr. Charlie Loke, to identify these uterine NK cells, which were later shown to be critical regulators of placental development.
Moffett’s research revealed that uterine NK cells help regulate the depth of EVT invasion, balancing the need for resource allocation between the parent and the fetus. Her team’s use of single-cell RNA sequencing in 2018 provided new insights, identifying three distinct subtypes of NK cells that each played a specific role in immune regulation and interaction with trophoblasts. These findings underscored the concept of pregnancy as a compromise, where both parent and fetus exert influence over resource exchange while maintaining an immunological boundary that supports coexistence.
Around the same time, breakthroughs in placental research included the development of trophoblast organoids, pioneered by Moffett’s lab and Martin Knofler’s team at the Medical University of Vienna. These organoids, three-dimensional cellular models, simulate certain aspects of placental development and function outside the body. By culturing cells from first-trimester placental tissue, researchers were able to replicate structures resembling villi, complete with hormone-secreting syncytiotrophoblasts (SCT) and migratory EVT, the two key cell types involved in placental development. Remarkably, these organoids produced human chorionic gonadotropin (hCG), a hormone detectable by home pregnancy tests, marking a significant step forward in creating models that reflect the in vivo environment. These "mini-placentas" offer a promising tool for studying placental diseases and testing treatments, shedding light on the intricate processes that sustain human pregnancy.
The studies by Moffett and others reveal that placental development is far from a passive process; it is a dynamic interplay of cell differentiation, immune regulation, and molecular signaling. This research helps demystify the complexities of pregnancy, transforming the view of the placenta from a simple organ of nutrient exchange into a sophisticated interface between two genetically distinct organisms, where survival depends on cooperation as much as competition.
The advances made by researchers like Ashley Moffett and Mariko Horii reflect a larger journey within reproductive and developmental biology, spanning decades of scientific progress, cultural shifts, and technological innovation. In the 1980s, when Moffett collected her rare hysterectomy samples, the concept of creating comprehensive cellular atlases was limited by the available scientific tools. Advances in genetic sequencing, spatial transcriptomics, and cellular imaging technologies have since enabled the detailed mapping of cell lineages and interactions that Moffett has now completed, shedding light on trophoblast differentiation—a critical process for healthy pregnancy.
One significant driver of progress in this field was the development of stem cell technology in the 1990s, led by pioneers like James Thomson. Thomson’s success in isolating human embryonic stem cells (hESCs) in 1998 opened new avenues for modeling human development. However, this advance raised ethical concerns that led to the development of induced pluripotent stem cells (iPSCs) in 2006. These iPSCs, created by reprogramming adult cells to behave like embryonic stem cells, offered a less controversial and more accessible resource for research. The ability to derive iPSCs from term placentas or umbilical cord cells has allowed researchers to model placental function and disorders, particularly preeclampsia, a complication characterized by poor trophoblast invasion and inadequate transformation of maternal blood vessels.
These stem cell-based models have bridged gaps left by earlier limitations. Prior to iPSCs and human trophoblast stem cells (hTSCs), researchers were restricted to using limited first-trimester tissue samples, cancer-derived cell lines, or non-human models, each with its drawbacks. Mouse trophoblasts, for instance, differ significantly from human trophoblasts in structure and gene expression, making them poor models for human placental development. However, the establishment of hTSCs and trophoblast organoids in 2018, combined with refined differentiation protocols, has allowed scientists to mimic key aspects of trophoblast behavior, such as hormone secretion and arterial remodeling, with far greater fidelity.
Horii’s work represents a critical evolution in placental research, addressing a longstanding challenge: modeling pregnancy complications. By using iPSCs derived from placentas affected by preeclampsia, her team has recreated hallmark abnormalities, including impaired oxygen sensing and defective trophoblast differentiation. These models can be further enriched with other placental and maternal cell types, such as blood vessels and stromal cells, providing a multi-faceted platform to study how these cells interact and contribute to disease.
The cumulative effect of these advances is a transformative toolkit for understanding and potentially mitigating pregnancy disorders. With these models, scientists can simulate the cellular environment of the placenta, manipulate conditions such as oxygen levels, and test therapeutic interventions in vitro. The promise of iPSC-derived trophoblast models and organoids lies not only in elucidating placental biology but also in offering targeted insights into conditions like preeclampsia, which remains a leading cause of maternal and fetal mortality worldwide. The research is poised to yield life-saving insights and, ultimately, treatments that could improve outcomes for mothers and babies, further exemplifying the profound impact of modern biotechnology on maternal health.
The placenta, often dismissed as a mere “support organ,” plays a vital role in human development, shaping not just the immediate health of the fetus but also the lifelong health of the individual. Formed soon after conception, the placenta is an intricate organ that attaches to the uterine wall, fostering a connection between mother and fetus. Its complex structure, particularly the highly convoluted syncytiotrophoblast (SCT) layer, provides an extraordinary surface area of approximately 13 square meters—akin to the size of a parking space. This vast surface allows for efficient nutrient, oxygen, and waste exchange, crucial for fetal growth. Despite its fleeting existence, this organ leaves a lasting biological imprint; studies increasingly show that placental health is closely linked to the future risk of diseases like diabetes, cardiovascular conditions, and obesity.
Historically, medical understanding of the placenta lagged behind other organs, partly due to the ethical and logistical challenges associated with studying it. The placenta is both elusive—functioning only during pregnancy—and complex, making it difficult to model accurately in laboratory conditions. In recent years, however, scientific advances have transformed placental research. Innovations such as trophoblast stem cells, which mimic the placenta’s primary cells, along with trophoblast organoids and placenta-on-a-chip models, are allowing researchers to better explore the placenta's dynamics and influence. These new tools, coupled with advanced imaging, gene editing via CRISPR, and high-resolution RNA sequencing, are providing unprecedented insights into placental function and development.
The placenta’s impact is not limited to the in utero period but has been linked to lifelong health outcomes. Disruptions in placental function can lead to preeclampsia, fetal growth restriction, and even miscarriage. This revelation has sparked renewed interest among researchers, who are now working to understand how the placenta’s in utero environment contributes to both typical and atypical pregnancy outcomes. These findings underscore the importance of further research and the need for robust models that can accurately simulate placental biology. With continued innovation, experts like Norah Fogarty are optimistic that, over the next few decades, the study of the placenta will advance to a level comparable with other organs, fostering improved prenatal care and potentially reducing the risk of chronic diseases for future generations.






















0 Comments